Background: Secondary hematologic malignancies are increasingly recognized due to the rising number of cancer survivors. While therapy related myeloid malignancies have distinct genetic profiles and inferior allogeneic stem cell transplantation (alloHCT) outcomes, there is limited data regarding secondary acute lymphoblastic leukemia (sALL). In one report, patients with therapy related ALL had an inferior OS of 23 months after alloHCT compared to primary (pALL) where OS was not reached [Saygin et al. 2019]. In the past decade, several novel therapies have been FDA approved for adults with B-ALL allowing more patients to achieve molecular remissions prior to alloHCT, therefore, we aimed to examine the role of alloHCT for adults with sALL compared to adults with pALL in a contemporary era.
Methods: We identified 247 (n= 25 sALL, n= 223 pALL) patients with ALL who underwent alloHCT at Moffitt Cancer Center from 1/2010 to 12/2022. Matching was performed with a 1:1 nearest neighbor matching on propensity score controlling for age, ALL subtype, MRD status, CR line at transplant, regimen intensity, and lines of therapy received for sALL. Three patients with sALL were excluded from matched analysis due to inability to control for selected covariates. Variable balance was assessed before and after matching. Outcomes measured included overall survival (OS), leukemia free survival (LFS), non-relapse mortality (NRM), and relapse. Survival analysis was evaluated by Kaplan-Meier estimations. Multivariate analysis was performed by Cox regression.
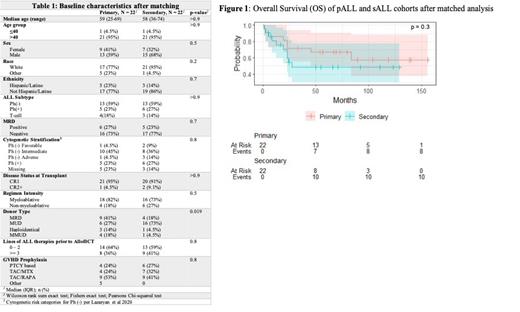
Results: After matching, 22 patients in the pALL group and 22 patients in the sALL group were analyzed. The two groups were evenly matched for all baseline characteristics except for donor type ( Table 1 p = 0.019). The majority of patients in both groups were over the age of 40, were diagnosed with Ph(-) B-cell ALL and were transplanted in first complete remission (CR1). Cytogenetic stratification for Ph(-) patients were defined per CIBMTR criteria [Lazaryan et al. 2020]. In patients with Ph(-) sALL, cytogenetics were classified as adverse (n=4), intermediate ( n=7), favorable (n=2) and were missing in 3 patients. In the sALL group, 59% of patients had an antecedent solid tumor malignancy whereas 36% had a hematologic malignancy with one patient having two prior malignancies. Half of the sALL group (n=11) had chemotherapy exposure for primary malignancy. Median follow up time for the pALL and sALL groups was 76 and 55 months respectively. Compared to the pALL group, the sALL group did not have a significant difference in OS ( Figure 1 p = 0.3). The 2 and 5-year OS of the pALL group was 72% and 67% respectively vs. 60% and 48% in the sALL cohort (p = 0.3). The 2 and 5-year LFS of the pALL cohort was 72% and 52% respectively vs 45% and 45% of the sALL cohort (p = 0.3). Cumulative incidence of NRM (27 % vs. 18%, p=0.7) and relapse (27% vs 32% p = 1.0) were not statistically significant between sALL and pALL groups. In a multivariate analysis performed after matching, there was no associated difference in the OS (p= 0.6) between groups.
Conclusion: In this study, we observed no significant difference in OS, LFS, NRM or relapse between sALL patients and pALL patients after controlling for key variables in a matched cohort analysis. Most patients included in this analysis were in complete molecular remission at time of transplant underscoring the prognostic impact of MRD. Despite 50% of the sALL cohort having exposure to prior chemotherapy, few patients had adverse cytogenetics suggesting that cytogenetic risk and MRD, and not only exposure to prior therapy are key prognostic factors for sALL patients undergoing alloHCT. Larger studies are needed to validate these findings. Despite small number of patients, we observe encouraging outcomes for this patient population with traditionally poor outcomes with conventional chemotherapy alone.
Disclosures
Elmariah:Bristol Myers Squibb: Research Funding. Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Jain:Incyte: Research Funding; Loxo@Lilly: Research Funding; Myeloid Therapeutics: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding. Shah:Pharmacyclics/Janssen, Spectrum/Acrotech, BeiGene, Gilead Sciences: Honoraria; DSMC, Pepromene Bio: Membership on an entity's Board of Directors or advisory committees; Celgene, Novartis, Pfizer, Janssen, Seattle Genetics, AstraZeneca, Stemline Therapeutics, Kite/Gilead: Other: Travel, Accommodations, Expenses; Incyte, Jazz Pharmaceuticals, Kite/Gilead, SERVIER: Research Funding; Moffitt Cancer Center: Current Employment; Takeda, AstraZeneca, Adaptive Biotechnologies, BMS/Celgene, Novartis, Pfizer, Amgen, Precision Biosciences, Kite/Gilead, Jazz Pharmaceuticals, Century Therapeutics, Deciphera, Autolus Therapeutics, Lilly, Pepromene: Consultancy. Bejanyan:Magenta Therapeutics: Consultancy; Medexus Pharmaceuticals: Consultancy; CTI BioPharma: Consultancy; CareDx Pharma: Consultancy; Orca Bio: Consultancy; Sanofi: Consultancy; AlloVir: Consultancy; CRISPR Therapeutics: Research Funding. Lazaryan:Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Faramand:Gilead: Research Funding; Kite: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal